New blood medicines arrive at all 3 end points and plan to apply for listing this year
May 04, 2018 Source: WuXi PharmaTech
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];Recently, Alexion Pharmaceuticals announced the positive results of Phase 3 in the development of the long-acting C5 complement inhibitor ALXN1210. The study showed that patients with paroxysmal nocturnal hemoglobinuria (PNH) can be effectively and safely converted to ALXN1210 treatment every eight weeks from every two weeks of Soliris (eculizumab) treatment.

PNH is a chronic, progressive, debilitating and potentially life-threatening ultra-rare blood disease that can occur in all races, backgrounds and ages of men and women without warning, with an average age of onset of 30 years old. . PNH is usually not recognized and delays range from 1 year to over 10 years. In PNH patients, chronic, uncontrolled immune system complement cascade activation leads to hemolysis, anemia, fatigue, dark urine, and shortness of breath. The most devastating consequence of chronic hemolysis is the formation of blood clots that can damage vital organs and even lead to premature death. One-third of patients with PNH are expected to survive five years after diagnosis. These patients urgently need effective therapy to prolong life.
Founded and developed by Alexion, ALXN1210 is an innovative long-acting C5 inhibitor that inhibits C5 Protein in the human immune system's complement cascade, preventing red blood cell damage and preventing PNH-induced clots and organ damage. The key difference between it and Soliris is that ALXN1210 is a long-acting preparation that is taken every eight weeks, while patients who use Soliris need to receive a drug infusion every two weeks.
In a clinical trial published in mid-March, ALXN1210 showed no worse than Soliris in patients with PNH (not treated with complement inhibitors). In this conversion study, ALXN1210 again confirmed the efficacy of not being inferior to Soliris, based on changes in lactate dehydrogenase (LDH) levels at the primary end point (a direct marker of complement-mediated hemolysis in PNH). The study also confirmed the non-inferiority of ALXN1210 at all four key secondary endpoints: the proportion of patients with breakthrough hemolysis, assessed by changes in the quality of life assessed by the Chronic Disease Therapy Function Assessment (FACIT)-Fatigue Scale, to avoid The proportion of patients who were transfused and who had stable hemoglobin levels. In addition, patients who used ALXN1210 did not develop breakthrough hemolysis, and 5 patients who used Soliris developed this condition. The detailed results of the study will be published at the upcoming medical conference.
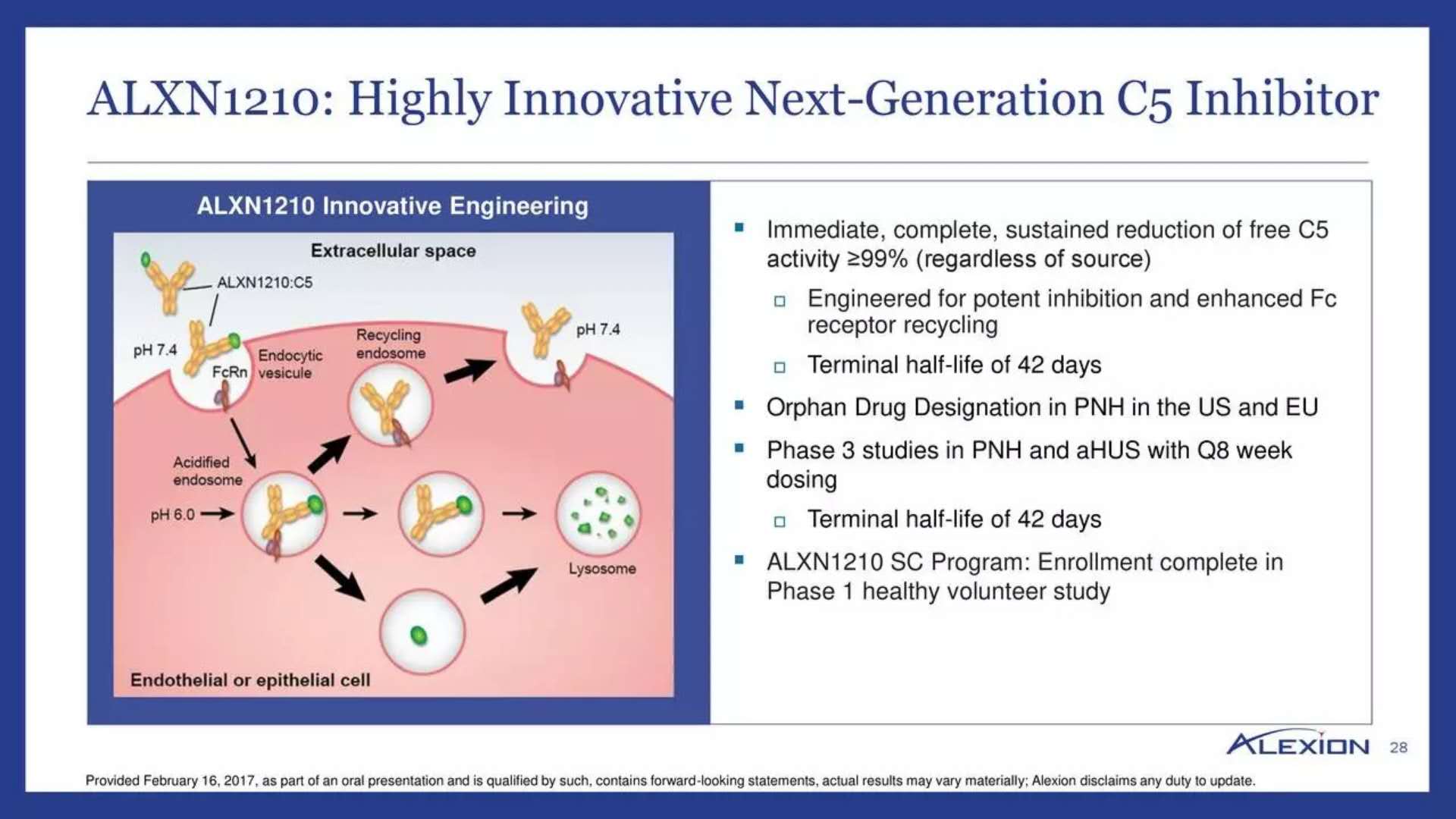
â–² ALXN1210 mechanism of action (Source: Alexion official website)
“ALXN1210 once again meets the high standards set by Soliris in the second large phase 3 study. Importantly, we now have strong data to prove that PNH patients can effectively and safely switch from Soliris to ALXN1210,†Alexion executed. Dr. John Orloff, vice president and head of research and development, said: "We are very pleased that more than 440 patients (including patients who have never received a complement inhibitor and patients who have been stabilized with Soliris and converted to ALXN1210) have a phase 3 PNH study. The overall data shows that all major and critical secondary endpoint outcomes support ALXN1210, including breakthrough hemolysis. We believe that the differentiated features of ALXN1210 may be meaningful improvements for patients and clinicians, and look forward to as soon as possible in the US in mid-2018 And the European Union submitted a global regulatory application, and then submitted a regulatory application in Japan later this year."
We expect this long-acting therapy to benefit PNH patients as soon as possible.
Reference materials:
[1] Alexion's Soliris successor clears phase 3, teeing up filings
[2] Alexion Reports First Quarter 2018 Results And Positive Topline Data From ALXN1210 Phase 3 PNH Switch Study
[3] WuXi PharmaTech - rare blood disease new drug phase 3 clinical data positive
Xi'an complex bio-tech CO.,LTD. , https://www.complexpowder.com